
However, efforts have been made to recycle lead-acid batteries and minimize environmental contamination from their disposal. These batteries are commonly used in vehicles, backup power systems, and various industrial applications.
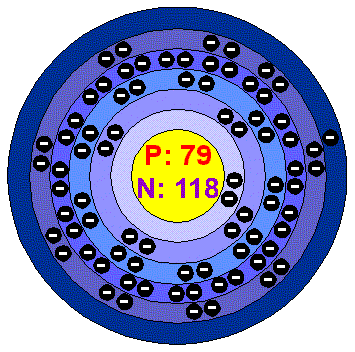
Lead has also been used extensively in the production of batteries, particularly lead-acid batteries. As a result, the use of lead pipes in plumbing has been phased out or replaced with safer alternatives, such as copper or plastic pipes. However, it is now widely recognized that lead can leach into the water, especially in older plumbing systems, and pose serious health risks if ingested. Lead pipes were commonly used for the transportation of water due to their durability and ease of shaping. One of the most well-known historical uses of lead is in plumbing systems. However, its use has significantly decreased in recent years due to its toxic effects on human health and the environment. Lead has been used by humans for thousands of years due to its malleability, low melting point, and corrosion resistance. It is a soft, dense, and bluish-gray metal with a variety of applications. Lead is a chemical element with the symbol Pb and atomic number 82. Some interesting facts of Lead are given below – Lead has density ‘11.35’ and it is found ”% on earth. Question : write some information about Lead ? Question : write the electron configuration of Lead element ?Īnswer : Lead electronic configuration is ” 4f14 5d10 6s2 6p2”. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.Answer : as we know Lead element is denoted by ‘Pb’ symbol and Lead has ‘207.2’ atomic mass and ’82’ atomic number. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.įinding molar mass starts with units of grams per mole (g/mol).
#LEAD ATOMIC MASS NUMBER HOW TO#
This site explains how to find molar mass. The reason is that the molar mass of the substance affects the conversion. To complete this calculation, you have to know what substance you are trying to convert.

These relative weights computed from the chemical equation are sometimes called equation weights.Ī common request on this site is to convert grams to moles. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.įormula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.


 0 kommentar(er)
0 kommentar(er)
